Documents review in eCTD process with ARender and Extedo.
Gain insights into how ARender and Extedo streamline the eCTD regulatory document review process, improving collaboration and efficiency in the pharmaceutical industry.
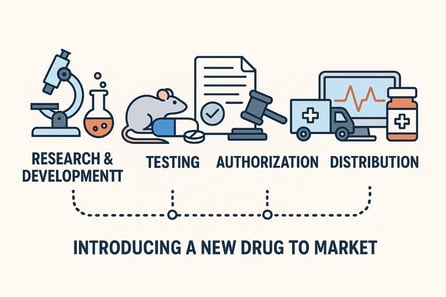
Introducing a new drug to the market is a long journey. After initial scientific advice and subsequent research and development, comes the evaluation of effectiveness—first on cells, then on animals, and finally through clinical trials. Once all of this is confirmed, the pharmaceutical company must obtain authorization for market introduction, before managing the complex distribution aspects and the continuous monitoring of the medicine’s impact.
Exploring the eCTD Submission Process
Last week, I gained a lot of insight into the middle step—“authorization”—as I was invited, along with Romain Monte, Product Manager of ARender (the embeddable information viewer and intelligent document processing service), to Frankfurt to attend eRA, e-Regulatory Affairs event organized by Extedo. Extedo is a partner who chose to embed ARender as the document viewing and interaction solution within their eCTD submission and review applications.
 First, what is eCTD? eCTD stands for Electronic Common Technical Document. It is an international standard, now in its fourth iteration, for submitting regulatory information to health authorities. It organizes and formats documents related to drug approval processes, enabling efficient review, tracking, and lifecycle management. eCTD ensures consistency, supports electronic submissions, and facilitates communication between pharmaceutical companies and regulatory agencies. (see visual from our partner Extedo).
First, what is eCTD? eCTD stands for Electronic Common Technical Document. It is an international standard, now in its fourth iteration, for submitting regulatory information to health authorities. It organizes and formats documents related to drug approval processes, enabling efficient review, tracking, and lifecycle management. eCTD ensures consistency, supports electronic submissions, and facilitates communication between pharmaceutical companies and regulatory agencies. (see visual from our partner Extedo).
Understanding the Scale of Document Review
The information submitted can be massive, depending on the case—often hundreds of documents and data points. There may be several rounds of back-and-forth between the industry lab and the regulatory agency. A single lab might submit tens or even hundreds of applications to get a given drug approved in various countries. And a lab can have thousands of drugs in its portfolio.
Now you follow me: multiple reviews of hundreds of documents that must comply with strict rules, repeated as many times as there are countries where you want to do business, and across the tens to thousands of drugs your group is working on. Multiply that by the hundreds of labs around the world, and you get an *immense* content management and document orchestration use case. This results in millions of highly specialized document reviews globally—all for the safety of the billions of people who rely on access to medicine. Wow. Even *wower* when you consider that agency resources are finite, while the pace of new drug development—boosted by GenAI in drug design—is accelerating.
How Extedo and ARender Add Value
As you can guess, my passion for automation got a little spark here ;-)
And this is where Extedo comes into play. They act as a bridge between labs and agencies, providing technology and solutions that bring peace of mind to labs by ensuring their submissions follow the highly regulated formats. They offer specialized solutions for authoring, submission, review by agencies, and even publishing after approval.
The event showcased the depth of Extedo’s portfolio, with many national and continental regulatory agencies attending, alongside labs and technology partners—including us.
Extedo chose to work with Uxopian Software to accelerate their roadmap by offloading complex UI developments. These include managing large case files, collaborative document browsing, annotations and sticky notes, and—one of the latest features ARender developed specifically for Extedo—support for hyperlinking. This allows reviewers to navigate all the content in a regulated sequence without ever leaving the viewer. The new publishing module was one of the major announcements at the event.
Real-World Feedback and What’s Next
Customer events are always a valuable opportunity for us to see how much value we bring to our partners. It’s also a chance to interact with their own customers and better understand how our product could evolve to meet their needs.
During the event, we discussed current limitations in our XFA (XML Forms Architecture) support, which we need to improve. Conversations also sparked interest in document comparison capabilities—as you can imagine, tracking changes is a major aspect of the review process—and how AI might support this effort. This was great timing, as our team is currently working on an AI-assisted comparison feature within ARender.
Looking Ahead: AI, FHIR, and Future Opportunities
Another interesting topic was the future of eCTD. There were differing views on whether the standard needs to evolve, but there was a clear consensus on the increasing importance of FHIR. FHIR (Fast Healthcare Interoperability Resources), developed by HL7, is a standard for the electronic exchange of healthcare information. It defines data formats, elements (called “resources”), and APIs for accessing and sharing clinical, administrative, and patient data. FHIR supports RESTful architectures and JSON/XML formats, promoting easy integration between modern healthcare applications. Think of it like CMIS—but with a much stronger data model enforcement.
I left the event thinking that a generic ARender connector for FHIR sources could be highly valuable to the industry!
A huge thank you to our customer and partner Extedo for inviting us to this meaningful and insightful event.
If you are a software vendor building vertically in a specific domain, do not hesitate to contact us to, like Extedo, accelerate your roadmap with document viewing, processing, collaboration and understanding capabilities !